Digitalisation for increased business benefit
With today’s ever higher demands on various aspects of production, it is important to find smart solutions that meet the customer’s expectations at the same time as increasing the flexibility of the in-house organisation.
CIM from Denmark is a leading software specialist within the Industry 4.0 concept. Working on the basis of proprietary structures and products, CIM develops complete, customised solutions that promotes making business-critical decisions founded on reliable data. The company’s customers, which are primarily active within the pharmaceutical and infrastructure sectors, use CIM’s solutions to quality-assure and improve the efficiency of their production lines.
Rapid, secure line clearance
The manufacture and handling of pharmaceuticals are subject to high demands as regards quality, cleanliness and documentation. It was previously common for each production line to run one specific product, but with today’s shorter production runs, several different products have to be able to be handled one after another in the same line. In order satisfy the demands associated with pharmaceutical production, extensive work takes place between different runs – line clearance. This process entails both physical cleaning of the previous run, as well as extensive documentation.
“A production line can be configured to handle several different products. When manufacturing pharmaceuticals, the line clearance process ensures that the equipment is completely free of the previous products before starting the next production round. This is important in order to prevent the products mixing and thereby being contaminated,” says Morten Kahr Nielson, Site Manager at CIM.
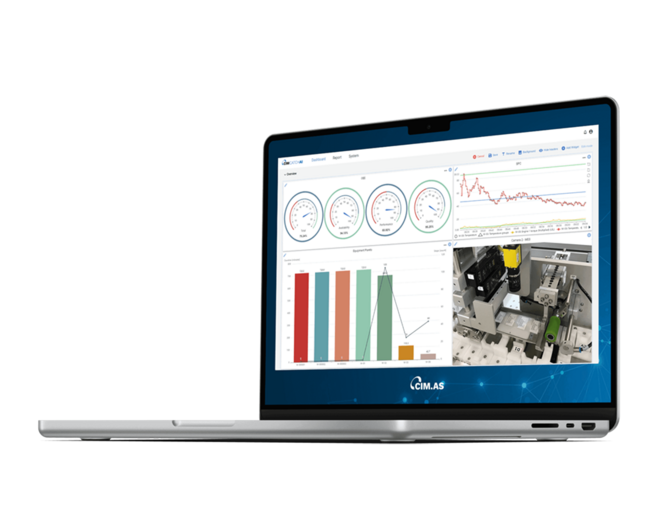
Time is a critical factor
During line clearance, time is a critical factor – every minute that the line is stationary means a loss of income. Working in close collaboration with customers, CIM has developed a digital solution that significantly shortens the set-up time, at the same time as safeguarding and documenting the process. With the time savings that are achieved, it is possible to make a return on the investment almost immediately.
“When manufacturing pharmaceuticals, as much as 75 per cent of the time is spent on documentation. You have to demonstrate that each stage of the production process is taking place in line with all regulations. This documentation is traditionally carried out manually, which means that it takes up a great deal of time. When line clearance is performed manually, there is also a risk of a particular element not being carried out correctly, which can lead to costly scrapping,” continues Morten Kahr Nielsen.
A solution with many benefits
The benefits of CIM’s solution are mainly financial, although optimised material consumption due to reduced waste and lower energy consumption are also factors. In addition, the burden of responsibility is also reduced for the operators. As well as minimising risk, flexibility is also increased as the tasks can be carried out by more employees when the specific knowledge requirements are not as extensive.

